How To Draw Binary Phase Diagram
A binary stage diagram shows the phases formed in differing mixtures of two substances as a role of temperature.
The
You determine the graph experimentally by measuring the cooling curves for various compositions.
Cooling Curves
If y'all absurd a liquid mixture containing about 67% lead and 33% tin (chiliad/chiliad), you lot become a cooling curve like that below.
Freezing starts at about 250°C.
Every bit the temperature drops, lead freezes out until, at 183°C, both tin can and pb are freezing.
Once everything has solidified, the temperature continues to fall.
This gives y'all two transition temperatures, one corresponding to
You repeat this process for various compositions, plot the pairs of points, and join them past smooth lines.
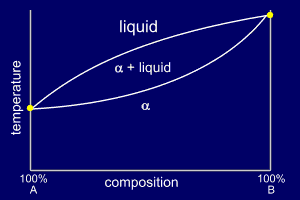
Solid Solutions
If the two substances, such equally metals, accept similar crystal structures, they often form solid solutions called alloys.
At each end of the phase diagram only pure
But at compositions in betwixt, y'all become two phase transitions.
The phase diagram usually looks like that beneath (α is the solid phase).
Eutectic Mixtures
Sometimes two substances can grade a mixture that freezes at a lower temperature than whatever other mixture of the components. This is chosen a eutectic mixture.
You use cooling curves every bit earlier, simply the phase diagram looks like that below.
Source: https://socratic.org/questions/how-can-i-construct-binary-phase-diagrams
Posted by: brownstered.blogspot.com


0 Response to "How To Draw Binary Phase Diagram"
Post a Comment